Características clínico-oftalmológicas y evolución de los pacientes con oftalmopatía tiroidea activa moderada severa tratados con bolos de metilprednisolona que acuden al Hospital Central del Instituto de Previsión Social, Paraguay
Palabras clave:
oftalmopatía tiroidea, oftalmopatía de Graves (OG), enfermedad de graves Basedow, glucocorticoides, metilprednisolonaResumen
Introducción: La oftalmopatía tiroidea (OT) es un trastorno debilitante en pacientes con enfermedad tiroidea autoinmune, principalmente enfermedad de Graves, que se desarrolla entre el 30 a 50% de los casos. Objetivos: Describir las características clínico-oftalmológicas y la evolución de los pacientes con oftalmopatía tiroidea activa moderada severa tratados con bolos de metilprednisolona que acuden al Hospital Central del Instituto de Previsión Social en el tiempo comprendido entre enero de 2018 y setiembre de 2021. Materiales y métodos: Investigación de diseño observacional, con estudio descriptivo, retrospectivo. Resultados:
Se revisaron fichas de 34 pacientes con OT activa moderada severa que recibieron bolos de metilprednisolona basado en las guías EUGOGO 2016, de los cuáles se excluyeron 3 pacientes por tener fichas incompletas y otros 3 pacientes ya que requirieron tratamiento de segunda línea previo al término del esquema de 12 sesiones. De los 28 pacientes estudiados, la edad promedio fue de 43,6 ±13,1 años, el 89% de sexo femenino y el 28,5%, fumadores. En cuanto a la función tiroidea de la población previo al tratamiento, se constató hipertiroidismo en el 82%, hipotiroidismo en el 11% y eutiroidismo en el 7%; y posterior al tratamiento, se
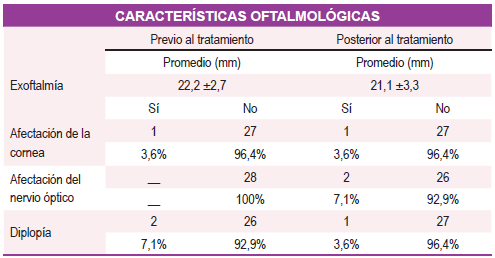
constató hipertiroidismo en el 78,6% (subclínico), eutiroidismo en el 17,9% e hipotiroidismo en el 3,5%. La mayoría (92.6%) contaba con anticuerpos contra el receptor de TSH positivo, con un promedio de 18 ± 9,9 mIU/Ml. Respecto a la actividad de la oftalmopatía según la escala CAS, se constató un promedio de 4,1 ±1,0 previo al tratamiento y posterior 1,2 ±1,4 de ellos el 46,4% presentó un estado leve según escala de gravedad, 39% sin criterios de gravedad y 14 % persistió en moderada -severa. Se constató mejoría de la agudeza visual tras el tratamiento (57,1%), el promedio de exoftalmía previo al tratamiento fue 22,2 mm y posterior 21,1 mm; se presentó diplopía en el 7,1% previo al tratamiento y en el 3,6% posterior al tratamiento. Conclusión: El tratamiento con glucocorticoides endovenosos en la oftalmopatía de Graves moderada-severa (esquema EUGOGO 2016) fue muy efectivo, revirtiendo la actividad y consecuentemente ayudando a disminuir la gravedad, en la gran mayoría de nuestros pacientes. Esto podría explicarse porque la oftalmopatía era incipiente y por el alto grado de adherencia de los pacientes en el contexto de un manejo multidisciplinar bien protocolizado.
Referencias
Edmunds MR, Boelaert K. Knowledge of Thyroid Eye Disease in Graves’ Disease Patients With and Without Orbitopathy. Thyroid Off J Am Thyroid Assoc. 2019;29(4):557-62.
Ariasgago V, Aguilar M, Weil D. Enfermedad ocular tiroidea. Oftalmol Clínica Exp [Internet]. 2021 [citado 17 de marzo de 2022];14(3). Disponible en: https://www.revistaoce.com/index.php/revista/article/view/70
Bartalena L, Piantanida E, Gallo D, Lai A, Tanda ML. Epidemiology, Natural History, Risk Factors, and Prevention of Graves’ Orbitopathy. Front Endocrinol. 2020;11:615993.
Torres Aquino HC. Características Clínico Epidemiológicas de Pacientes con Hipertiroidismo Atendidos en el Consultorio de Endocrinología del Hospital Hipólito Unanue de Tacna Periodo 2014- 2018 [Internet] [Tesis de Grado]. [Tacna-Perú]: Universidad Privada de Tacna; 2019 [citado 17 de marzo de 2022]. Disponible en: http://repositorio.upt.edu.pe/handle/20.500.12969/785
Nicolí F, Lanzolla G, Mantuano M, Ionni I, Mazzi B, Leo M, et al. Correlation between serum anti-TSH receptor autoantibodies (TRAbs) and the clinical feature of Graves’ orbitopathy. J Endocrinol Invest. 2021;44(3):581-5.
Khong JJ, Finch S, De Silva C, Rylander S, Craig JE, Selva D, et al. Risk Factors for Graves’ Orbitopathy;the Australian Thyroid-Associated Orbitopathy Research (ATOR) Study. J Clin Endocrinol Metab. 2017;101(7):2711-20.
Urquía-Sequeiros OA, Álvarez A, Segovia-Palomo A. Oftalmopatía de Graves eutiroidea con anticuerpos negativos. Rev Mex Endocrinol Metab Nutr. 2021;8:104-7.
Kahaly GJ. Management of Graves Thyroidal and Extrathyroidal Disease: An Update. J Clin Endocrinol Metab. 2020;105(12):dgaa646.
Dolman PJ. Grading Severity and Activity in Thyroid Eye Disease. Ophthal Plast Reconstr Surg. 2018;34(4S Suppl 1):S34-40.
Galindo-Ferreiro A, Marqués-Fernández VE. Orbitopatía tiroidea. Revisión sobre puntos clave para el diagnóstico y tratamiento [Internet] [Tesis de Especialidad]. [España]: Universidad de Salamanca; 2021 [citado 17 de marzo de 2022]. Disponible en: https://gredos.usal.es/handle/10366/148888
Pagano Boza C, Ortiz-Basso T, González Barlatay J, Ugradar S, Russo Picasso MF, Fassi J, et al. Prevalencia de enfermedad ocular tiroidea en un hospital argentino. Oftalmol Clínica Exp [Internet]. 2020 [citado 17 de marzo de 2022];13(4). Disponible en: https://www.revistaoce.com/index.php/revista/article/view/33
Rachwani-Anil R, Zamorano-Martín F, Rocha-de-Lossada C, García-Lorente M, Pérez-Casaseca C, Hernando-Ayala C, et al. Enfermedad inflamatoria orbitaria idiopática. Arch Soc Esp Oftalmol. 2022;97(2):89-99.
Längericht J, Krämer I, Kahaly GJ. Glucocorticoids in Graves’ orbitopathy: mechanisms of action and clinical application. Ther Adv Endocrinol Metab. 2020;11:2042018820958335.
Mora Botia GL. Tratamiento médico con agentes biológicos para la enfermedad inflamatoria orbitaria idiopática: revisión bibliográfica [Internet] [Tesis de Especialidad]. [España]: Universidad de Valladolid; 2020 [citado 17 de marzo de 2022]. Disponible en: https://uvadoc.uva.es/handle/10324/42601
Gómez C, Abad Rubio A, Taboada LB, Henao DC, Marín LF, Camargo J, et al. Orbitopatía tiroidea: protocolo de manejo basado en revisión de la evidencia. Rev Colomb Endocrinol Diabetes Metab. 2019;6(3):210-7.
Le Moli R, Malandrino P, Russo M, Lo Giudice F, Frasca F, Belfiore A, et al. Corticosteroid Pulse Therapy for Graves’ Ophthalmopathy Reduces the Relapse Rate of Graves’ Hyperthyroidism. Front Endocrinol [Internet]. 2020 [citado 17 de marzo de 2022];11. Disponible en: https://www.frontiersin.org/article/10.3389/fendo.2020.00367
Pereira Kiappe O. Corticosteroides no tratamento da orbitopatia de Graves: revisão sistemática de ensaios clínicos randomizados [Internet] [Tesis de Especialidad]. [Botucatu]: Universidade Estadual Paulista; 2019 [citado 16 de marzo de 2022]. Disponible en: https://repositorio.unesp.br/handle/11449/183113
Godfrey KJ, Kazim M. Radiotherapy for Active Thyroid Eye Disease. Ophthal Plast Reconstr Surg. 2018;34(4S Suppl 1):S98-104.
Kotwal A, Stan M. Current and Future Treatments for Graves’ Disease and Graves’ Ophthalmopathy. Horm Metab Res Horm Stoffwechselforschung Horm Metab. 2018;50(12):871-86.
González-García A, Sales-Sanz M. Tratamiento de la oftalmopatía de Graves. Med Clínica. 2021;156(4):180-6