Introduction
A basic group in the
security, food sovereignty and cultural identity of the Andean countries is the
one made up of tubers, among which are “ibias” or “ocas” (Oxalis tuberosa Molina),
“cubios”, “nabos” or “majuas” (Tropaeolum tuberosum Ruiz & Pav.),
“chuguas” or “ullucos” (Ullucus tuberosus Caldas) and potatoes (Solanum
tuberosum L.), all with a wide diversity in the inter-Andean valleys
(Clavijo-P. et al., 2014; García et al., 2018; Viteri et al., 2020).
Oxalis tuberosa has been used by Andean populations for thousands of years, it
has an adaptation to environments where other crops cannot survive, including
tolerance to cold climates, thrives in soils with pH of 5.3 to 7.8, resistant
to various pests and other phytosanitary problems (Rosero, 2010; Clavijo-P. &
Pérez-M., 2014).It is probable that this species or some related ones, have
been consumed and perhaps cultivated by the inhabitants of the inter-Andean
valleys since the Holocene (Clavijo-P. et al., 2014). Rosero-A. (2010)
identified the south of the department of Nariño, especially the municipalities
of Cumbal, Guachavéz, El Encano and Puerres, as the area of greatest diversity
of this species in the department. Although the species is a material that propagates vegetatively
cycle after cycle, there is evidence of variation at the phenotypic and
genotypic level that should be conserved and used for the generation of new and
better materials that respond to the interests of farmers, producers and
consumers (Morillo et al., 2016; Clavijo-P. y Pérez-M., 2014), but conserving
native germplasm to avoid its loss.
The Tropaeolum tuberosum,
like O. tuberosa thrives in cold climates and is tolerant of various
types of pests and diseases, but its taste is not very liked by the population
due to its strong bitter taste caused by the high content of isothiocyanates
derived from glucosinolates (Grau et al., 2000) which has decreased its
consumption, despite presenting high nutritional and very possibly medicinal
value (Leidi et al., 2018). Two possible domestication centers are proposed for
this species, the highlands located between Peru and Bolivia and the Cundiboyacense
savanna in Colombia, where varieties with dissimilar morphological and
physiological characteristics are found (Grau et al., 2003; Tapia &
Fries,2007). However, Seminario (2004), considers that the tuberization
phenomenon is related to adaptations to areas with prolonged periods of
drought, rare phenomena in the Colombian region. Manrique et al. (2014) identified the micro-centers of
diversity by morphological characterization of 107 accessions; finding that the
states of Huancavelica, Junín, Pasco and Cusco represent 62% of all Peruvian
accessions (Manrique et al., 2014). In the state of Cusco, Ortega et al.
(2007), analyzed the patterns of genetic diversity of cultivated and wild
populations of T. tuberosum, a wide genetic diversity was found, and it
was determined that wild populations are closely related to cultivated ones.
These results strengthen the Cusco region as a micro center of diversity of T.
tuberosum.
Ullucus tuberosus
and T. tuberosum have been shown
to have more than 35% starch, along with high resistance to pests, low
temperatures and drought (Campos et al., 2018; Naranjo et al., 2017). Tapia &
Fries (2007) consider that one of the domestication centers of U. tuberosus is
Colombia, since the cultivated morphotypes present wild characteristics such as
creeping growth and smaller diameter of the tubers. However, Parra-Q et al.
(2012) suggest that the site of origin was in the central Andes, between Peru
and Bolivia, from where it was dispersed semi-domesticated towards Colombia and
later the erect growth morphotypes were domesticated that had a new wave of
dispersal towards the north. In Colombia, in the departments of Boyacá and
Cundinamarca, 36 accessions were morphologically and molecularly characterized
and it was concluded that the populations of the center of the country
correspond to the subspecies in the process of domestication, given its decumbent
growth and its genetic distance with the populations of the south of the
country (Parra-Q. et al., 2012).
Regarding potatoes, it is
widely accepted that one of the main centers of initial domestication of
potatoes is in southern Peru on the border with Bolivia 10,000 years ago
(Hawkes & Ruel, 1989). From the domesticated species and through
hybridization with other species of the genus, the other species of the section
would have been obtained Petota. Another center for domestication of
potatoes is the island of Chiloé in Chile, where wild and domesticated species
of Solanum have been recorded (Tapia & Fries 2007; Ovchinnikova et
al., 2011, Cadima & Terrazas, 2019). According to Gómez et al.
(2012), Colombia is the center of origin and domestication of Creole potatoes, S.
phureja. Navarro et al. (2010) morphologically and molecularly
characterized 19 genotypes of S. tuberosum and S. phureja in
Nariño, Colombia; however, it was found that neither morphological nor
molecular markers can clearly distinguish the evaluated species and varieties.
In fact, Huamán & Spooner (2002) morphologically characterized eight
species of potatoes in Latin America and concluded that given the genetic
plasticity and continuous hybridization, all the evaluated potatoes could well
be classified as a single species, S. tuberosum, with eight different
cultivars.
As can be seen, the high
varietal and intravarietal diversity of these species has been promoted and
accumulated after thousands of years of selection and management by traditional
Andean farmers. However, factors such as the homogenization of agroecosystems,
cultural uprooting and the globalization of the diet, endanger the future
maintenance of this diversity (Malice & Baudoin, 2009); in the nineties
Stephen Brush (Brush et al, 1992), and those of Karl Zimmerer, carried out
several studies where they identified the factors that led to the incorporation
of new varieties, but they also highlighted that some groups of peasants sought
the conservation of native species, almost thirty years later Velásquez-M et al.
(2016). They also point out the need to apply various actions for conservation,
including the identification of agrobiodiversity zones at the local, regional
and national level, to avoid genetic erosion.
The distribution of the
diversity of Andean tubers is not homogeneous but is identified concentrated in
micro-centers of diversity and outside of them less interspecific diversity is
conserved: various authors have identified as micro centers of diversification
of these: Cajamarca, Huancavelica, Huánuco and Cusco in Perú; in Bolivia the
Altiplano and La Candelaria territories; in Ecuador the provinces of Carchi and
Huaconas and in Colombia in the departments of Boyacá and Nariño (García &
Cadima, 2003; Seminario, 2004; Ortega et al., 2007; Parra-Q. et al. 2012;
Manrique et al. 2014, Clavijo-P. et al. 2014; Fonseca et al., 2014). These
micro-centers of diversification share a high intravarietal diversity, both
morphological and genetic, as well as an important cultural roots of these
species. The latter is possibly due in part to the fact that they occupy the
same agroecological niche and the farmer generally sows them in association or
in very close plots with similar management (Tapia, 2000).
Panbiogeography is a
geographic approach applied to contribute to the understanding of biodiversity
by identifying biotic components, individual traces, generalized traces and
panbiogeographic nodes, starting from the distribution of plant and animal
species (Morrone, 2004), which was proposed by Leon Croizant in 1952. Based on
the coordinates of the presence of a certain taxon, lines are constructed that
connect all the points so that the sum of the segments is the smallest possible
(individual trace or minimal laying tree); generalized traces result from the
superposition of individual traces of a phylogenetically related group;
panbiogeographic nodes are defined as the nodes of the generalized lines and
can be interpreted as areas where there is a greater presence of endemisms,
greater phylogenetic diversity and geographic limits of taxa (Miguel-T
& Escalante, 2013).
These methods have been used
to analyze the distribution of wild species (Martínez-G. & Morrone, 2005;
Arana et al.,2013; Morrone, 2013), but they have not been used to understand
the distribution of cultivated species, considering that the distribution
patterns of cultivated plants are not only governed by natural evolutionary
dynamics but especially by anthropic dynamics.
The objective of this study is to identify for the tuberous species of the
Andes, the agrogeographic nodes, thus named to the points that imply the
intersection of sociocultural and biogeographic histories, which can have the
following interpretations: presence of unique local cultivars, absence of
widely distributed varieties, high inter- and infra-specific diversity and agro-ecosystemic
affinities between areas. To compare the microcenter nodes reported in the
bibliography with the agro-geographic nodes, the generalized line resulting
from intersecting the individual lines of the Andean tubers will be estimated (S.
tuberosum, O. tuberosa, U. tuberosus and T. tuberosum). In
this way it will be possible to identify potential areas of concentration of
the diversity of Andean tubers not previously reported. The identification of
these areas will guide research on the conservation and management of Andean
tubers. In addition, the agro-geographic nodes shed elements of discussion on
the exchange of seeds and knowledges between indigenous peoples.
Materials
and Methods
A database was built of the
distribution of O. tuberosa, U. tuberosus, T. tuberosum and
S. tuberosum in: Chile, Argentina, Bolivia, Perú, Ecuador, Colombia, and
Venezuela, from the records integrated into the global geographic information
platform, Global Biodiversity Information Facility (Global Biodiversity
Information Facility [GBIF], 2017). Individuals which presented coordinates of
complete geographic distribution, latitude, longitude, state and municipality
were taken into account.
According to panbiogeographic
theory, the distribution coordinates of each species were individually
connected in such a way that the distance between the points was minimal,
taking into account the curvature of the Earth (Miguel-T. & Escalante,
2013). This procedure was performed with the program PASSaGE 2.14.3 ‘Essen’
(Rosenber & Anderson, 2011) and Quantum-GIS 2. (Sherman et al., 2007),
applying the combined method (Liria, 2008), which combines spatial analysis
through geodetic distance calculation, creating a connectivity matrix and
minimum spanning trees, with layer management and GIS spatial operations of
intermediate storage and interception, thus the individual traces obtained from
each species intersect each other to obtain the generalized traces and the agro-geographic
nodes.
Analogously to the main biogeographic nodes
(Morrone, 2015a), the main agro-geographic nodes correspond to the areas where
there are more points of intersection of the analyzed species and the secondary
ones also represent important areas of diversity but of lesser magnitude. The
main nodes, in terms of wild species, identify the areas where speciation
phenomena possibly occurred; while for cultivated species they would indicate
initial domestication phenomena, from where they would distribute the seeds to
other areas where they would continue their domestication.
Results
A number of 8975 records had
the required geographic information. The species with the highest abundance is S.
tuberosum with 6056 records distributed in Peru (3088), Argentina (943),
Bolivia (783), Colombia (502), Ecuador (410), Chile (294) and Venezuela (36);
regarding infraspecific taxa, it was found that the subspecies S. tuberosum
andigena (Juz. & Bukasov) Hawkes y Roel
(2006) cuenta con mayor número de registros (3758), Solanum
stenotomum Juz. & Bukasov (456), Solanum phureja Juz. &
Bukasov (359), Solanum chaucha Juz. & Bukasov (352) y los registros
restantes corresponden a otras variedades y formas de S. tuberosum L (GBIF,
c2017).
For its part, O. tuberosa
had 1400 records distributed in Peru (1056), Bolivia (146), Argentina (111),
Ecuador (63), Chile (13) and Colombia (11) (GBIF, c2017).
U. tuberosus presented 1010 records; 736 in Peru, 137 in Ecuador, 83 in
Bolivia, 38 in Argentina and 16 in Colombia (GBIF, 2017).
Table 1. Accessions of species in relation to their geographical origin.
|
Perú
|
Argentina
|
Bolivia
|
Colombia
|
Ecuador
|
Chile
|
Venezuela
|
Total
|
|
S. tuberosum
|
3088
|
943
|
783
|
502
|
410
|
294
|
36
|
6056
|
|
O. tuberosa
|
1056
|
111
|
146
|
11
|
63
|
13
|
|
1400
|
|
U. tuberosus
|
736
|
38
|
83
|
16
|
137
|
|
|
1010
|
|
T. tuberosum
|
350
|
6
|
29
|
24
|
100
|
|
|
509
|
|
Totales
|
5230
|
1098
|
1041
|
553
|
710
|
307
|
36
|
8975
|
|
|
|
|
|
|
|
|
|
|
Finally, T. tuberosum was
registered 509 times, 40 of them correspond to T. tuberosum silvestre
Sparre and the remaining 469 to T. tuberosum Ruiz & Pav.; Regarding
the distribution by countries, Peru reports 350, Ecuador 100, Bolivia 29,
Colombia 24 and Argentina six (Global Biodiversity Information Facility [GBIF],
2017).
Andean tubers are distributed
in the biogeographic regions of the Transition Zone of South America, provinces
of Páramo, Puna, Desierto, Monte, Maule, Santiago and Valdivia, and from the
Neotropical region in the provinces of Yungas, Rodonia, Ucayali, Cauca,
Ecuatorian, Western ecuadorian, Magdalena, Sabana and Chaco. The provinces with
the highest number of records are Yungas for S. tuberosum with 2241, while in
the province of Puno the highest number of records is reported for U.
tuberosus (453), O. tuberosa (805) and T. tuberosum (166);
the western Ecuadorian and Sabana provinces are the ones with the fewest
records with a single report for T. tuberosum and O. tuberosa,
respectively (Figure 1).
Current distribution maps and
individual traces of each of the species were obtained (Figure 1). These lines
converge in a generalized line that runs from the north of Argentina to the
center of Colombia, crossing through Bolivia, Peru and Ecuador.
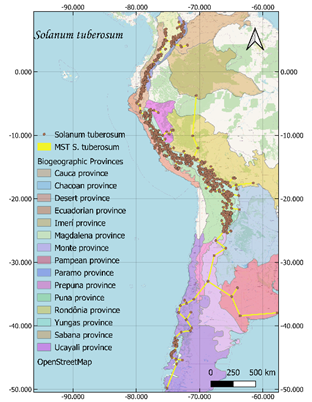
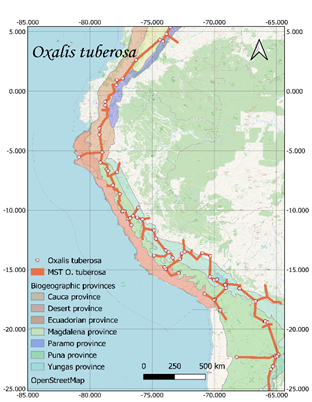
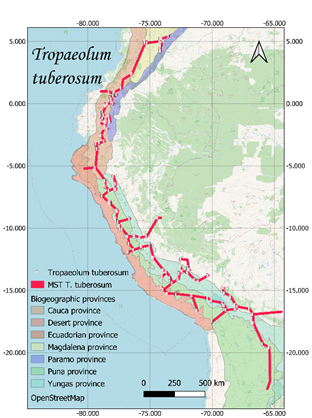
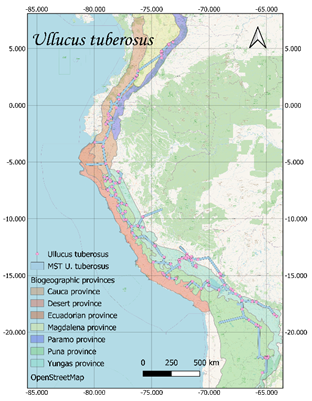
Figure 1. Distribution and minimum laying tree of S. tuberosum, O.
tuberosa, U. tuberosus and T. tuberosum on biogeographic provinces
(Loöwenberg-Neto, 2014) and Open Street Map.
The main node of this trace,
or primary agro-geographic node occurs in southern Peru, a secondary node was
identified in the north of this country and several secondary nodes were found
in Bolivia, in central Peru, in central Ecuador and on the borders between
Colombia and Ecuador (Figure 2). Regarding the biogeographic
distribution, the number of nodes is greater in the southern Brazilian domain,
followed by the South American Transition Zone and the Pacific domain.
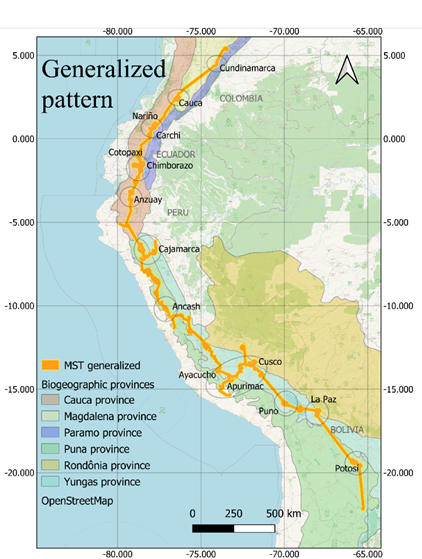
Figure 2.
Generalized trace and agrogeographic nodes (in circles) of Andean tubers on
Biogeographic Provinces (Löwenberg -Neto, 2014). and Open Street
Map.
In Peru, 1,245 of the 2,043 generalized or intersection
coordinates were identified (61%). The main node is between the departments of
Cusco (277), Apurímac (111) and Ayacucho (89) and in second order are the nodes
of Cajamarca (181), Ancash (134) and Puno (103) that stand out for the number
of intersection points of individual traces.
In Ecuador, 459 sites with
intersection coordinates (22.5%) were found, located mainly in the provinces of
Chimborazo (123), Cotopaxi (61), Carchi (51) and Anzuay (42).
In Bolivia there were 204
sites with intersection coordinates (10%), identified in the departments of La
Paz (109) and Potosí (95); the municipalities of Tomás Frías (83) Pedro Domingo
Murillo (39) and Manco Kapac (34) were the ones that presented the highest
abundance of intersection coordinates in the country.
In Colombia, 131 sites with intersection
coordinates (6.5%) were identified, in the departments of Cauca (46), Nariño
(37), Cundinamarca (28) and Boyacá (19), mainly. In Nariño, the municipalities
of Cumbal (16) and Ipiales (6) were the most representative; in Cauca they were
Totoró (18) and Silvia (11); in Cundinamarca the municipalities with the
highest abundance are Chipaque (11), Bogotá (5), Pasca (5) and Ubaque (4);
Finally, in Boyacá, the municipality of Samacá (12) was the one that presented
the highest abundance.
Discussion
According to biogeographic
theory, panbiogeographic nodes imply the intersection of different ecological
and biogeographic histories that can have the following interpretations:
presence of local endemisms, absence of widely distributed taxa, high
phylogenetic diversity and geographic affinities with other areas and
boundaries or geographic or phylogenetic of taxa (Miguel-T & Escalante,
2013; Morrone, 2015b; Grehan, 2020). In this analysis, the identified nodes do
not correspond to panbiogeographic nodes but to agro-geographic nodes,
understanding that human beings have been decisive in the flow of seeds of
cultivated plants, as well as in the selection and adaptation of their
varieties (Velásquez et al., 2013).
Molecular studies confirm the
highlands of Peru, departments of Huánuco, Pasco, Junín, Huancavelica,
Apurímac, Ayacucho, Cusco and Puno as the domestication region of S. tuberosum;
where, due to temperature fluctuations, the development of plants with growth
of underground propagules is favored (Morales-G., 2007). The first presence of
potatoes in Peru dates back to 6900 years ago in the department of Lima and
towards the beginning of the Formative period (3,800 years ago) in the
department of Junín (Morales-G, 2007). Spooner et al. (2005) suggest that the
domesticated potato comes from the wild species of the S. brevicaule
complex from southern Peru, although this complex extends from Huánuco to Puno.
Currently in the region of Cusco, Apurímac and Huancavelica it is common to
find conservationist farmers for whom biodiversity is their lifestyle and
therefore they grow 50, 100 or 300 varieties of potatoes in plots smaller than
one hectare (Fonseca et al., 2014).
The Cusco region is also an
important area for the conservation of the other Andean tubers. T. tuberosum
registers a greater genetic and morphological diversity in the agroecosystems
of this area than that conserved in the collection of the International Potato
Center (Manrique et al., 2014). O. tuberosa it also presents a high
diversity in the departments described, as well as in Cajamarca, Ancash and
Puno (Pissard et al., 2008). Currently, the exchange between Andean tuber seed
farmers has been reported between the Peruvian departments of Huánuco,
Huancayo, Huancavelica, Ayacucho, Cajamarca and Cusco (Velásquez et al., 2013).
In Bolivia, the departments
of La Paz and Cochabamba have been reported as micro-centers of diversity of
Andean tubers in this country (García & Cadima 2003). Cochabamba is one of
the regions with the greatest morphological and molecular diversity of O.
tuberosa (Emshwiller & Doyle, 1998), T. tuberosum (Grau et al.,
2003) and U. tuberosus (Parra-Q., 2012).
The Ecuadorian departments of
Carchi, Cañar, Bolívar, Chimborazo, Cañar, and Loja, present a wide diversity
of potatoes (Morales-G 2007), ullucos (Vimos et al., 1993) and Mashuas (Grau et
al., 2003). In Colombia, the use of lithic tools has been reported for more
than 5000 years as hoes, the edges of which have residues of starch from tubers
(Aceituno-B & Rojas-M, 2012). The southeastern part of the country, mainly
the municipality of Cumbal and others in the department of Nariño, are
recognized micro-centers of potato diversity (Tinjacá-R. & Rodríguez-M.,
2015) and ullucos (Parra-Q., 2012). In the department of Boyacá, the
municipality of Ventaquemada has been identified (Clavijo-P. et al., 2014) as
one of the Colombian micro-centers. However, in our analysis the municipality
of Samacá was also identified as the administrative unit where the intersection
coordinates in the department concur with greater abundance. The department of
Cauca was also identified, especially the municipalities of Totoro and Silvia,
as those with the greatest presence of coordinates where the four species are
present in the department; however, studies have not yet been carried out to
determine the varietal diversity of these resources. The same occurs in the
department of Cundinamarca, where the municipality of Chipaque has the highest
abundance of intersection coordinates but does not have associated studies.
Biological diversity is
concomitant to areas where there is also a high cultural diversity as a whole,
they make up biocultural diversity, a key diversity in the processes of plant
domestication in Mesoamerica and the Andes (Casas et al., 2017). In this sense,
the identified nodes concur in areas of indigenous predominance: Quechua
(Bolivia, Peru, Ecuador, and Colombia), Aimara (Peru and Bolivia), Pastos
(Ecuador and Colombia), Nasa, Misak and Coconucos (Colombia); With the
exception of the node in central Colombia where only remnants of the Muisca
indigenous group remain (Albo et al., 2009).
The panbiographic analysis of Andean tubers offers two benefits,
namely: First, it allows the identification of the geographic areas with the
greatest agrobiodiversity and this in turn is important to direct protection
and recovery strategies for plant genetic resources. On the other hand, it also provides evidence in the
reconstruction of the history and diffusion of domestication in America,
allowing temporal trans-scalarity, that is, connecting the interpretation of
past events with current processes (Casas et al., 2017). The results support
the hypothesis that Andean tubers were domesticated in the Peruvian highlands,
from where they dispersed to the south and north following the patterns of
pre-Columbian human occupations. In this sense, the history of the Andean tubers
can be understood as the history and future of the Andean peoples (Viteri et
al., 2020; Devaux et al., 2021; Montes et al., 2021).
Author
contributions
Conceptualization: R.F.G.-D, E.F.V.-H,
L.M.-C. Experiment design: R.F.G.-D, E.F.V.-H., L.M.-C.,
F.J.D.-N., S.A.-S. Experiment execution: R.F.G.-D, E.F.V.-H. Experiment
verification: R.F.G.-D, E.F.V.-H.,
L.M.-C., F.J.D.-N., S.A.-S. Data
analysis/interpretation: R.F.G.-D., E.F.V.-H., L.M.-C., F.J.D.N.,
S.A.-S. Statistical analysis: R.F.G.-D, E.F.V.-H., L.M.-C., F.J.D.N.,
S.A.-S. Manuscript preparation: R.F.G.-D,
E.F.V.-H., L.M.-C., F.J.D.-N., S.A.-S. Manuscript editing and revision: R.F.G.-D, E.F.V.-H., L.M.-C.,
F.J.D.-N., S.A.-S. Approval of the final
manuscript version: R.F.G.-D, E.F.V.-H., L.M.-C., F.J.D.-N.,
S.A.-S.
Financing
This study was supported by the “Corporación
Universitaria Minuto de Dios (Dirección General de Investigaciones y Centro
Regional Zipaquirá)” by funding this research within the framework of the
project C116-085 "Andean tubers back home: participatory conservation of
tuberous species in Cundinamarca".
References
Aceituno-B. F. &
Rojas-M. S. (2012). Del peleoindio al formativo: 10000 años para la historia
lítica en Colombia. Bol. Antropol., 26(43), 124-156.
Albo, X., Argüelles, N., Ávila,, R., Bonilla, L. A., Bulkan, J.,
Callou, D., Carriazo, C., De Castro, A. F., Censabella, M., Crevels, M., Díaz,
M., Couder, E. D., García, F., Haboud, M., Hernández, A., Leite, Y., Koskinen.,
A., Lemus, J., López, L. E., … & Trillos, M. (2009). Atlas
sociolingüístico de pueblos indígenas en américa latina. Bolivia. [UNICEF]
Fondo de las Naciones Unidas para la Infancia, [FUNPROEIB] Fundación para la
Educación en Contextos de Multilingüismo y Pluriculturalidad, Andes. 522 p.
Arana, M., Ponce, M., Morrone, J. &
Oggero. A. (2013). Patrones biogeográficos de los helechos de las sierras de córdoba
(Argentina) y sus implicancias en la conservación. Guayana
Bot., 70(2), 357-376.
Brush, B. S., Taylor, J. E. &
Bellón, M. R. (1992). Technology adoption and biological diversity in andean
potato agriculture. J. Dev. Econ., 39(2), 365-387.
Cadima X. & Terrazas, F. (2019). Caracterización de los
semilleristas tradicionales de papa en Bolivia. Revista Latinoamericana de
la Papa, 23(1), 56 – 62
Campos, D., Chirinos, R.,
Gálvez-Ranilla, L. & Pedreschi, R. (2018). Bioactive
potential of andean fruits, seeds, and tubers. Advances in food and
nutrition research, 84, 287-343.
Casas, A., Parra, F., Torres-G., I., Rangel-L., S., Zarazúa M.
& Torres-G J. (2017). Estudios y patrones continentales de domesticación y
manejo de recursos genéticos: perspectivas. En:Casas, A., Torres-G., J. &
Parra-R. editores. Domesticación en el continente americano. México.
Universidad Nacional Autónoma de México, Universidad Nacional Agraria la Molina
del Perú. 538-563.
Clavijo-P., N. L. & Pérez-M.
M. E. (2014). Tubérculos andinos y conocimiento agrícola local en comunidades
rurales Ecuador y Colombia. Cuadernos de desarrollo rural, 11(74),
149-166. doi:10.11144/Javeriana.CRD11-74.taca.
Clavijo-P., N. L., Barón, M. T.
& Combariza, J. A. (2014). Tubérculos andinos: conservación y
uso desde una perspectiva agroecológica. Bogota.
Pontificia Universidad Javeriana.
Devaux, A., Hareau G., Ordinla,
M., Andrade-Piedra, J. & Thiele G. (2021). Las papas nativas: de ser un
cultivo olvidado al boom culinario e innovación de mercado. Revista
Latinoamericana de la Papa, 25 (2), 3–14. doi: 10.37066/ralap.v25i2.429.
Emshwiller, E. & Doyle, J. (1998). Origins of
domestication and polyploidy in oca (Oxalis tuberosa: oxalidaceae):
nrdna its data. Am J Bot., 85(7), 975–985.
doi:10.2307/2446364.
Fonseca, C., Rodríguez, F., Munoa, L. & Ordinola M. (2014).
Catálogo de variedades de papa nativa con potencial para la seguridad
alimentaria y nutricional de apurímac y huancavelica. Perú. Centro
Internacional de la Papa. doi:10.4160/9789290604549.
García, R. F., Jiménez, L., Bernal P. S., Robayo, O., Chaparro, M.
D., Sierra F. M., Camacho, P.A., Vanegas, J., Rincón, J. S. & León-S, T. E.
(2018). Tubérculos andinos de vuelta a casa: conservación participativa de
tubérculos andinos en cundinamarca. Bogotá D.C., Corporación Universitaria
Minuto de Dios.
García, W. & Cadima, X., editores. (2003). Manejo
sostenible de la agrobiodiversidad de tubérculos andinos: síntesis de
investigaciones y experiencias en bolivia. Conservación y uso de la
biodiversidad de raíces y tubérculos andinas: Una década de investigación para
el desarrollo (1993-2003). Bolivia. Fundación para la Promoción y la
Investigación de Productos Andinos PROINPA, Alcaldía de Colomi, Centro
Internacional de la Papa ClP, Agencia Suiza para el Desarrollo y la Cooperación
COSUDE.
Global Biodiversity Information
Facility [GBIF]. (2017). http://www.gbif.org
Gómez-P., T. M., López-O, J. B., Pineda-T., R., Galindo-L., L. F.,
Arango-I. R. & Morales-O, J. G. (2012). Caracterización citogenética de
cinco genotipos de papa criolla, Solanum phureja (juz. Et buk.). Rev.
Fac. Nac. Agron. Medellín., 65(1), 6379–6387.
Grau, A., Ortega-D., R., Nieto-C., C. & Hermann M. (2003). Mashua
Tropaeolum tuberosum Ruiz and Pav. Promoting the conservation and use of
underutilized and neglected crops. 25. Italy. International Potato Center,
Lima, Perú/ International Plant Genetic Resources Institute.
Grau, A., Ortega, R., Nieto, C. & Hermann, M. (2000). Promoting
the conservation and use of underutilized and neglected crops. Mashua
(Tropaeolum tuberosum Ruiz and Pav,). Rome,
Italy. IPGRI, 54p.
Grehan, J. (2020). Conserving
biodiversity as well as species. BSO Newsletter 80, 14-18
Hawkes, C. & Ruel, M. T.
(2006). Understanding the links between agriculture and health. Washington:
International Food Policy Research Institute.
Hawkes, J. G. (1989). The
domestication of roots and tubers in the american tropics. In Harris, D.R.,
Hillman, G.C., editors. Foraging and farming. The evolution of plant
exploitation. Unwin Hyman, London, 481-503.
Huamán, Z. & Spooner, D. M.
(2002). Reclassification of landrace populations of cultivated potatoes (Solanum
sect. Petota). Am J Bot. 89(6),947–965. doi:10.3732/ajb.89.6.947.
Leidi E.O, Monteros-Altamirano,
A., Mercado, G., Rodríguez, J. P., Ramos, A., Alandia, G., Sorenses, M. &
Jacobsen, S.E. (2018). Andean roots and tubers crops as
sources of functional foods. J. Funct. Foods., (51),
86-93. doi: 10.1016/j.jff.2018.10.007.
Liria J (2008). Sistemas de
información geográfica y análisis espaciales: un método combinado para realizar
estudios panbiogeográficos. Revista Mexicana de Biodiversidad 79: 281-
284.
Löwenberg-Neto, P. (2014). Neotropical region: a shapefile of Morrone's
(2014) biogeographical regionalisation.
Zootaxa,
3802 (2), 300-300
Malice, M. & Baudoin, J.P. (2009).
Genetic diversity and germplasm conservation of three minor andean tuber crop
species. Biotechnol Agron Soc Environ., 13(3),
441–448.
Manrique, I., Arbizu, C., Vivanco, F., Gonzales, R., Ramírez, C.,
Chávez, O., Tay, D. & Ellis, D. (2014). Tropaeolum
tuberosum Ruíz & Pav. Colección de germoplasma de mashua conservada en
el Centro Internacional de la Papa (CIP). Lima.
CIP. doi:10.4160/9789290604310.
Martínez-G. M. & Morrone, J. (2005). Patrones de endemismo y
disyunción de los géneros de euphorbiaceae sensu lato: un análisis
panbiogeográfico. Bol. Soc. bot. Méx., 77, 21-23.
Miguel-T., C. & Escalante, T. (2013). Los nodos: el aporte de
la panbiogeografía al aporte de la biodiversidad. Biogeografía, 6,
30-42.
Montes, J. M., Daza, L. F. &
Angarita, L. M. (2021). Productos andinos para el desarrollo de una gastronomía
nacional. Sosquua, 2(2), 59-69.
Morales G., F. J. (2007).
Sociedades precolombinas asociadas a la domesticación de la papa Solanum
tuberosum en Sudamérica. Revista Latinoamericana de la Papa, 14(1),
1-9.
Morillo, C.A., Morillo, C. Y.
& Tovar, L.Y. (2016). Caracterización molecular de cubios (Tropaeolum
tuberosum Ruíz y Pavón) en el departamento de Boyacá. Rev
.Cienc. Agric., 33(2), 32–42.
doi:10.22267/rcia.163302.50.
Morrone J. (2015b). Track analysis
beyond panbiogeography. J. of Biogeogr, 42, 413-425.
Morrone, J. (2004). Panbiogeografía,
componentes bióticos y zonas de transición. Rev. Bras.
Entomol., 48(2), 149-162.
doi:10.1590/S0085-56262004000200001.
Morrone, J. (2015a).
Biogeographical regionalisation of the andean region. Zootaxa, 3936(2),
207-236. doi:10.11646/zootaxa.3936.2.3.
Morrone, J. J. (2013). Cladistic
biogeography of the Neotropical region: identifying the main events in the
diversification of the terrestrial biota. Cladistics: 1-13. http://10.1111/cla.12039
Naranjo Quinalusa, E. J., Tapia
Bastidas, C. G., Velázques Feria, R. J., Cruz Péres, Y., Delgado Pilla, A. H.,
Borja Borja, E. J. & Paredes Andrade, N. J. (2017). Caracterización
eco-geográfica de melloco (Ullucus tuberosus) En la región alto andina
del ecuador. Revista agro ciencias, (19)
Navarro, C., Bolaños, L. C. y
Lagos, T. C. (2010). Caracterización morfoagronómica y molecular de 19
genotipos de papa guata y chaucha Solanum tuberosum L. y Solanum
phureja Juz et Buk cultivados en el Departamento de Nariño. Revista de
Agronomía. 27 1 27–39.
Ortega, O. R., Duran, E., Arbizu,
C., Ortega, R., Roca, W., Potter, D. & Quiros, C. (2007). Pattern
of genetic diversity of cultivated and non-cultivated mashua, Tropaeolum
tuberosum, in the Cusco region of Perú. Genet Resour Crop Evol. 54(4),
807–821. doi:10.1007/s10722-006-9160-y.
Ovchinnikova, A., Krylova, E.,
Gavrilenko, T., Smekalova, T., Zhuk, M., Knapp, S. & Spooner, D. M. (2011).
Taxonomy of cultivated potatoes (Solanum section petota: solanaceae).
Biol. J Linnean. Soc., 165(2):107–155. doi:10.1111/j.1095-8339.2010.01107.x
Parra-Q., M., Panda, S, Rodríguez,
N. & Torres, E. (2012). Diversity of uUlucus tuberosus
(basellaceae) in the colombian andes and notes on ulluco domestication based on
morphological and molecular data. Genet Resour Crop Evol., 59(1), 49–66.
doi:10.1007/s10722-011-9667-8.
Pissard, A., Arbizu, C., Ghislain,
M., Faux, A. M., Paulet, S. & Bertin, P. (2008). Congruence between
morphological and molecular markers inferred from the analysis of the
intra-morphotype genetic diversity and the spatial structure of Oxalis
tuberosa mol. Genética, 132(1):71–85. doi:10.1007/s10709-007-9150-9.
Rosenberg, M.S. & Anderson, C.
D. (2011). Passage: pattern analysis, spatial statistics and geographic
exegesis. Version 2. Methods in Ecology and Evolution, 2(3), 229-232.
Rosero-A., M. G. (2010). Colección,
caracterización y conservación de variabilidad genética de oca (oxalis tuberosa
mol) en agroecosistemas paramunos del departamento de nariño-colombia. (Tesis
Maestría). Palmira. Universidad Nacional de Colombia.
Seminario J. (2004). Origen de
las raíces andinas. En Seminario J., editor. Raíces Andinas: Contribuciones
al conocimiento y a la capacitación. Serie: Conservación y uso de la
biodiversidad de raíces y tubérculos andinos: Una década de investigación para
el desarrollo (1993-2003) Nº 6. Lima. Universidad Nacional
de Cajamarca, Centro Internacional de la Papa y Agencia Suiza para el
Desarrollo y la Cooperación.
Sherman, G. E.; Sutton, Blazek,
R.; Holl, S.; Dassau, O.; Mitchell, T.; Morely, B. & Luthman, L.. (2007).
Quantum GIS ver.0.8 ‘TITAN’.
Spooner, D. M., McLean, K.,
Ramsay, G., Waugh, R., Bryan, G.J. (2005). A single domestication for potato
based on a multilocus amplified fragment length polymorphism genotyping. Proc Natl
Acad Sci U S A, 102,14694-14699. doi: 10.1073/pnas.0507400102.
Tapia M.E. 2000. Cultivos andinos
subexplotados y su aporte a la alimentación (Versión 1.0) Santiago de Chile. FAO.
http://www.fao.org/tempref/GI/Reserved/FTP_FaoRlc/old/prior/segalim/prodalim/prodveg/cdrom/contenido/libro10/home10.htm
Tapia, M. E. (1997). Cultivos
andinos subexplotados y su aporte a la alimentación (Version 1.0). Santiago de
Chile. FAO. https://bibliotecadigital.infor.cl/handle/20.500.12220/3020
Tapia, M. E. & Fries, A. M. (2007). Guía de campo de los
cultivos andinos. Lima: FAO y ANPE.
Tinjacá-R., S. & Rodríguez-M., L. E. (2015). Catálogo de
papas nativas de nariño, Colombia. Bogotá: Universidad Nacional de
Colombia.
Velásquez-M., D., Casas, A., Torres-G., J. & Cruz-S., A.
(2016). Erosión genética de las comunidades andinas. En Casas A., Torres-G., J.
& Parra, F., editores. Domesticación en el continente americano. 1, 97-131.
Velásquez, D., Trillo, C., Cruz, A. & Bueno S. (2013).
Intercambio tradicional de semillas de tuberosas nativas andinas y su
influencia sobre la diversidad de variedades campesinas en la sierra central
del Perú (Huánuco). Zonas Áridas., 15(1), 110-127.
doi:10.21704/za.v15i1.111.
Vimos, N., Nieto, C. & Rivera, M. (1993). El melloco,
características, técnicas de cultivo en ecuador. Ecuador. Instituto
Nacional Autónomo de Investigaciones agropecuarias.
Viteri, C., Camino, M., Robayo, D., Moreno, T. & Ramos, M.
(2020). Alimentos sagrados en la cosmovisión andina. Revista Ciencia e Interculturalidad,
27(02), 173-189. https://doi.org/10.5377/rci.v27i02.10442

![]() DOI: 10.57201/IEUNA2313312
DOI: 10.57201/IEUNA2313312![]() , Universidad
Nacional de Asunción.
, Universidad
Nacional de Asunción.![]() , Centro
de Desarrollo e Innovación Tecnológica (CEDIT)
, Centro
de Desarrollo e Innovación Tecnológica (CEDIT)![]()
![]() , Edna Fabiola
Valdez-Hernández2
, Edna Fabiola
Valdez-Hernández2![]() *, Leonardo
Martínez-Cárdenas3
*, Leonardo
Martínez-Cárdenas3![]() , Francisco
Javier Díaz-Nájera4
, Francisco
Javier Díaz-Nájera4![]() y Sergio Ayvar-Serna4
y Sergio Ayvar-Serna4![]()




