Flores Lugo, J. A.,
Flores García, A. L., Torres Portillo, M. D., Avila Mascareño, M. F., Cota
García, K. P., Martínez Vidales, A. D., Parra Cota, F. I. & Santos
Villalobos, S. S. (2022). Celulosa bacteriana: una alternativa promisoria para
diversificar la producción de papel de manera sostenible. Revista investigaciones y estudios – UNA, 13(2), 59-75.
https://doi.org/10.47133/IEUNA22206b
Abstract. Global environmental changes related to
anthropogenic activities are becoming increasingly common. Currently, there are
multiple environmental problems caused by human production systems, which
require more sustainable alternatives to reduce and/or mitigate their negative
impact on the environment, highlighting activities such as tree felling.
Vegetable cellulose (obtained mainly from trees) is a raw material of great
importance in manufacturin of paper; therefore, the future of forests depends
on the manufacture and consumption of paper. The present review analyzes the
use of bacterial cellulose as a raw material for substituting vegetable
cellulose in the paper industry for a possible reduction of tree felling.
Keywords. bacterial cellulose, papermaking, biotechnology, environmental
issues.
Resumen. Los
cambios ambientales globales relacionados con las actividades antrópicas son
cada vez más comunes. En la actualidad, existen múltiples problemas ambientales
provocados por los sistemas de producción humanos, los cuales, requieren de
alternativas más sostenibles para reducir y/o mitigar su impacto negativo en el
medio ambiente, destacando actividades como la tala de árboles. La celulosa
vegetal (obtenida principalmente de los árboles) es una materia prima de gran
importancia en la fabricación de papel; por lo que el futuro de los bosques
depende de la fabricación y el consumo de papel. La presente revisión analiza
el uso de la celulosa bacteriana como materia prima para la sustitución de la
celulosa vegetal en la industria papelera para una posible reducción de la tala
de árboles.
Palabras clave. celulosa bacteriana, fabricación del
papel, biotecnología, problemas ambientales.
INTRODUCCIÓN
Currently, it is
possible to observe diverse environmental problems around us, such as
deforestation, which is carried out for multiple purposes, primarily for paper
manufacture. For centuries humans have used fibrous raw material to make paper.
(Area, 2008). The paper and related products are made from cellulose, the main
component of plant cell walls. This cellulose can come from various plants,
such as cotton, wood, cereal straw, and sugar cane, among others (Area, 2008),
although most papers are made from cellulose from wood (Lera Santín, 2011).
Trees are of major environmental importance since they host many
species (birds, insects, mammals); in addition to photosynthesis, providing
oxygen, and absorbing carbon dioxide. Furthermore, they help regulate the
planet's climate and prevent soil erosion. Deforestation is a severe problem
and one of the leading causes of habitat destruction, causing biodiversity
loss, and negative changes in carbon dioxide (CO2) fixation. In
deforesting regions, the soil is affected by erosion and is frequently degraded
to non-productive lands. Considering all these negative environmental impacts,
there is a promising solution, which consists of using bacterial cellulose
obtained from microorganisms. Bacterial cellulose, like plant cellulose, is an
insoluble extracellular polymer that is produced by a variety of bacterial
species within the genera Achromobacter, Agrobacterium, Rhizobium, Sarcina,
Zoogloea, and Gluconacetobacter (Chávez-Pacheco et al., 2004). Gluconacetobacter
xylinus (formerly Acetobacter xylinum) is a Gram-negative bacterium,
strictly aerobic, that performs incomplete oxidation of several sugars and
alcohols (a process known as oxidative fermentation). Its natural ecological
niche is decomposing fruits and vegetables, and it can produce bacterial
cellulose on liquid and solid media by forming a film on the surface (Chávez-Pacheco
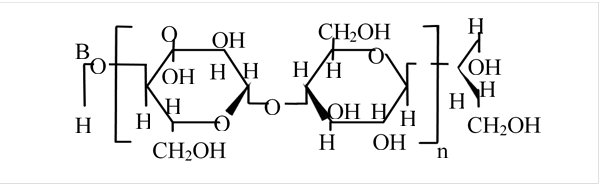
et al., 2004). The cellulose synthesized by G. xylinus is
structurally and chemically the same as the plant cellulose; however, the
bacterial cellulose is found with high purity, free of lignin and
hemicellulose, and has a high capacity for water absorption and mechanical
resistance. Cellulose fibers synthesized by bacterial cellulose are much
smaller than wood fibers (Chávez-Pacheco et al., 2004). These peculiarities
make bacterial cellulose a feasible material for papermaking.
Conventional paper manufacturing
In papermaking
there are multiple production methods, previously the most popular was
mechanical abrasion and the application of chemicals based on caustic soda,
sulfites, and sulfates. Currently, the most widely used methods are the
mechanical and the Kraft method (based on sodium hydroxide and sodium sulfide)
(Boeykens, 2006; Mues et al., 2018).
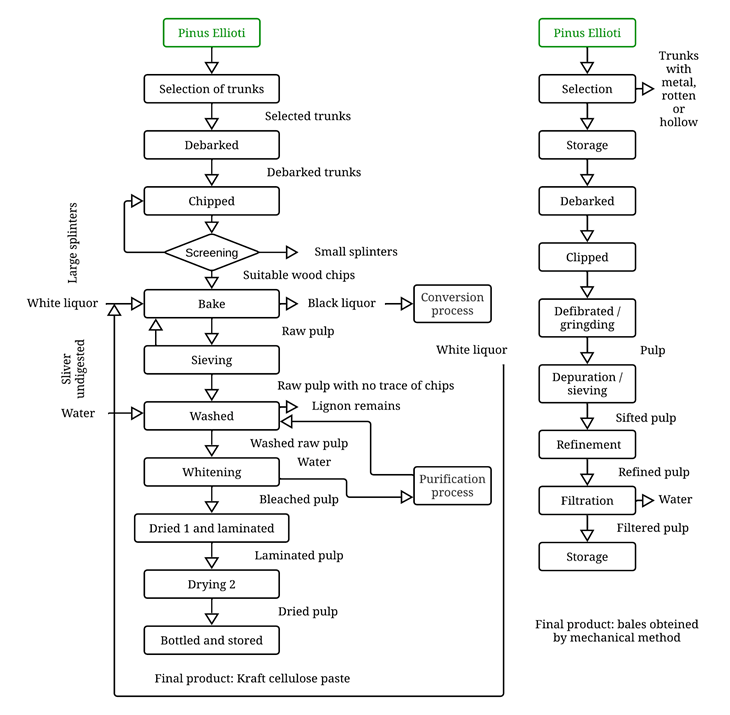
The paper-making
process begins with the treatment of raw material by debarking and shredding
the wood to obtain the cellulose. Then, the pulp is prepared, depending on the
type of tree it came from: i) chemical treatment in which the wood previously
converted into small chips is passed through a cooking process with chemical
products, for example, the kraft treatment for resinous trees of Evergreen,
currently one of the most used (Boeykens, 2006), covering approximately 72% of
the world's cellulose production, this process generates a large amount of
waste that is difficult to biodegrade and has use of 60% (Mues et al., 2018);
and ii) mechanical process for trees of expired leaf, this method represents
20% of world production, which requires significant energy consumption and its
use is 90 to 98% to obtain pulp (Mues et al., 2018). The composition of the
wood is complex. Cellulose is the cell wall's basic structural element and is
accompanied by lignin and hemicellulose.. It gives it a dark brown coloration,
for that reason, it requires a bleaching process. In this stage can be used
different bleaching agents like hydrogen peroxide (which changes the structure
of lignin altering the color, but does not eliminate it), chlorine,
hypochlorite, or chlorine dioxide, which generates toxic waste, in addition,
the treatment of water after bleaching (Boeykens, 2006; Mues et al., 2018;
Merizalde et al., 2019). At this point the cellulose pulp has high water
content, so it is deposited in a machine where it is transferred on a long belt
driven by rollers, then, the water is removed by different procedures, like
gravity, vacuum, pressure, and drying. And finally, a massive sheet of paper is
obtained, which is rolled up to form a bovine (Figure 1) (Mues et al., 2018).
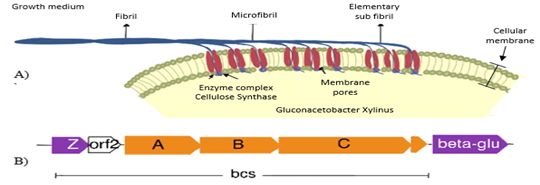
The function of
the bcsC gene and the bcsD gene has not yet been discovered. However, it is
proposed that the bcsC gene functions as a 3.5 nm pore-forming protein, which
is diametrically aligned in the cell membrane to allow cellulose secretion. In
contrast, the bcsD gene appears to be involved in controlling the
crystallization process of cellulose nanofibrils (Liu et al., 2018b). Besides,
it is hypothesized that the bcsD gene is found extracellularly and that its
function is to twist the newly synthesized cellulose fibrils, generating
higher-order fibers, which would explain why G. xylinus produces
fibrillar cellulose and other bacteria that lack the bcsD gene produce
amorphous cellulose (Prada Ramírez, 2014).
Recently, Kubiak
et al., (2014) identified the orf2 whose function is still unknown, and the
bcsZ gene that encodes an endo-β-(1-4)-glucanase
whose function is to divide the bacterial cellulose chains, consequently, more
chains are generated and when these are joined microfibrils are formed.
Finally, located downstream (3') of the cellulose synthase operon there is a
gene that encodes a β-glucosidase,
which hydrolyzes glucose units formed by more than three monomers and plays an
important role in cellulose production and the microfibril packaging produces
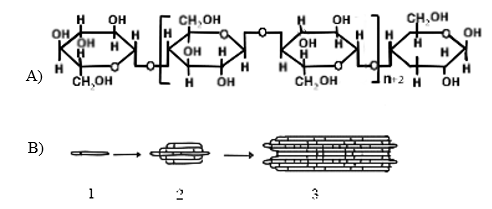
the cellulose fibers and ribbons (Figure 4) (Schmid et al., 2015) which reach a
thickness of 1 to 9 µm and form an extensive reticulated structure stabilized
by hydrogen bonds, the condensation of the tapes gives rise to the
three-dimensional structure or macrostructure of the bacterial cellulose
(Gorgieva & Trček ,2019).
Production of bacterial cellulose
The production of
bacterial cellulose strictly depends on the growth medium, which can be static
or agitated. Furthermore, it influences the sources of carbon, nitrogen, pH,
temperature, availability of oxygen, minerals, the proportion of the inoculant,
and finally the age that the inoculum present (Santos et al., 2016; Aswini et
al., 2020). In the CB production industry, it is common to use molasses
obtained from sugar cane as a carbon source. In addition, different additives
are used to increase its production (Table 5). These additives can be glycerol,
sodium alginate, ethanol, etc. among others (Gorgieva & Trĉek, 2019).
Currently, there
are different methods to produce bacterial cellulose, some of them can be
obtained through shaken, static cultures or grown in bioreactors (Wang et al.,
2019). When static media are used, there is usually a uniform cellulose layer
i.e., a film is formed between the liquid and air phase of the culture, the
purpose of cellulose synthesis is to supply a firm surface matrix, which allows
the microorganism, a strictly aerobic, to be close to the gas phase (Carreño Pineda
et al., 2012); in the case of shaken cultures or agitation, cellulose usually
appears in the form of clusters or masses in an irregular manner (Tuya Salas et
al., 2021). The production of bacterial cellulose through the use of agitated
cultures is more advisable for the industry since a greater production is
obtained (due to greater availability of oxygen), however, this technique
usually presents a low level of polymerization and has a higher crystallization
characteristic when compared to static culture (Lahiri et al., 2021; Gorgieva
& Trĉek, 2019).
Reactors used in the production of
bacterial cellulose
As mentioned,
bacterial cellulose can be produced by a) bioreactors called airlift and b)
trickling bed.The first (a) presentssufficient oxygen with a reduced feed
source, resulting in cellulose in pelletized form. The second (b) has a high
availability of oxygen but at a decreased shear strength, where the product can
be found in irregular leaves (Gorgieva & Trĉek, 2019).On the other hand,
there is a so-called rotating disk reactor, which consists of a series of
circular disks mounted on the horizontal axis that rotate and are alternately
exposed to the fermentation environment and the air. The cellulose obtained in
the reactor does not face strong shear stress and has excellent oxygen
transmissibility, so microorganisms can easily come into contact with it. Thus,
the production of bacterial cellulose by Gluconacetobacter xylinus was
maximized at 5.52 g/L; In addition, constant aeration improve production,
reaching a production of 5.67 g/L (Guinea Nava, 2016).
Valuation of
trees, water pollution, and soil los
Carbon dioxide (CO2)
is the primary anthropogenic greenhouse gas (GHG) in the atmosphere, it
contributes approximately 65% to the radiative forcing produced by long-lived
GHGs (National Oceanic and Atmospheric Administration [NOAA], 2016).
Of the total emissions
from human activities during the period 2006-2015, about 44% were stored in the
atmosphere, 26% in the oceans, and 30% on land. One way to reduce the effects
of CO2 is to store it in the biomass through photosynthesis,
considering that the potential for carbon capture by plant communities varies
according to their structure and the constitution of each ecosystem (Jiménez Pérez
et al., 2020)
Since the end of
the of the last century, Central American countries carbon fixation and capture
capacity have been evaluated to measure their potential for the mitigation of
Greenhouse Gases (GHG) caused by growing industrial activity (López et al.
2018). Forest plantations have become a strategic means of fixing atmospheric
CO2 in the plant structure. In the paper industry, the prices of a
ton of fixed C vary between $ 4.6 - 50.4 US, depending on the actual discount
rates and the amount of C added to that obtained in the traditional wood
business (Gutiérrez Velez & Lopera Arango, 2001).
The absorption
potential of the forestry sector in Mexico has been estimated at 58 million
tons of carbon dioxide equivalent (t CO2e) by 2020 and 96 million tons of CO2e
by 2030 (Coordinación General de Producción y
Productividad de la Comisión Nacional Forestal [CONAFORT],
2013). For Mexico, in 2014, 137.8 million hectares of forest area were counted,
representing 70.5% of the 196.5 million hectares of the national territory.
México wood production is insufficient to satisfy the internal demand. In 2016,
6.71 million m3 of round wood was produced (29% of the total
volume), with an apparent consumption of 23.4 million m3. Of the
6.71 million m3 of wood, different classes of wood products were
obtained where cellulose represents a total of 0.61 million m3
(9.2%); however, from 1997 to 2016, a decreasing trend of cellulose was
registered with an average annual rate of -3.50% (Centro
de Estudios para el Desarrollo Rural Sustentable y la Soberanía Alimentaria [CEDRSSA], 2019). Even so, a representative amount of the country's
forest area continues to be deforested, with 9.2% going to the production of
this cellulose.
20% of the wood
used in papermaking comes from original natural forests and 29% from
plantations, and 54% from secondary forest (Greenpeace, 2004). Plantation
forests are increasing rapidly in the world to alleviate deforestation and
degradation of natural forests, along with providing goods and services.
Monoculture plantations have been the dominant type of plantation in practice
and are well-recorded in research, a higher diversity of tree species increases
the number of ecological niches, which can further increase the number of
associated species, loss of soil productivity and fertility, disruption of
hydrological cycles, risks associated with plantation forestry practices (e.g.,
the introduction of exotic species), risks of promoting pests and diseases,
higher risks of adverse effects of storms and fire, and negative impacts on
biodiversity (Liu et al., 2018b).
In the paper
industry, in addition to the problem of deforestation, there is high
consumption of water, energy, and chemical substances that can lead to the
contamination of water sources and air (Romero Conrado et al., 2017).
Water is one of the key components of papermaking; paper production is
unthinkable without water. The pulp and paper industry is ranked as the world’s
third-largest water consumer and produces high amounts of wastewater. In 2019,
the total use of water in Spanish pulp and paper mills stood at 111 million m3
per year, which is half the water of water than in 1990 and 30% less than in
2000, while production has increased by 60% since the beginning of the nineties
and 22% since the beginning of the XXI century (Carpintero, 2019). It is worth
mentioning that the paper industry has improved its processes in the last few
decades and as a result, has been able to reduce its water consumption
significantly. For the last several years the paper industry has been working
to reduce its freshwater consumption and its wastewater output. Reuse is an
important approach for reducing wastewater generation and consumption of
wastewater, however, the process is still carried out mainly from water from
rivers 69%, from wells 19% and only from the network, and recovered water 12% (Carpintero,
2019).
Thanks to the
voluntary agreement signed between the Ministry of the Environment and the
Association of Pulp and Cardboard Manufacturers (ASPAPEL) during the years
2000-2009, marked a fundamental milestone in the efficiency of water use and discharges
in the sector, achieving a 37% reduction in the use of water per ton produced
(from 24.5 m3/t to 15.5 m3/t) and a 28% reduction in the use of total water
(going from 160 cubic hectometers/year to 115 cubic hectometers/year), despite
an increase in production of 14% in the same period. To reduce water
consumption, the Spanish paper industry requires a powerful investment effort
(almost 1,400 million euros of investment in the last four years alone), to
reduce environment effects(Carpintero, 2019).
The importance of
soil losses can be focused on the services it provides, like “filtering of
nutrients and contaminants”, the economic cost numbers based on estimations of
nitrate unit damage, based on the soil retention and the unit damage cost, were
in the range 0–280 id$ ha-1 year-1. For the service of
“climate regulation” the total average cost of reducing greenhouse gas
emissions under the Mitigation Scenarios is estimated at between €190 and €240
tons of CO2 (2008 prices), and for “biomass production” the grassland,
shrubland, and pasture cost 740 id$ha-1 year-1
(270–1580); non-irrigated arable land 2230 id$ha-1 year-1
(440–4730); fruit and berry plantations cost 7500 id$ha-1 year-1
(6100–7580); and Olive groves 10,810 id$ha-1 year-1
(6540–10,910) (Jónsson et al., 2017). Soil can have or provide other important
goods and services, maintenance is, therefore, essential to achieve
environmental and economic sustainability.
Comparison of paper production
costs with plant cellulose vs. bacterial cellulose
Bacterial
cellulose represents the purest form of cellulose, and it possesses several
unique properties. Despite its impressive potential for a wide range of
commercial applications, bacterial cellulose is prohibitively expensive to
manufacture. This high cost limits its use as an alternative to plant
cellulose. The synthetic media commonly used for this kind of cellulose
production are the major factor contributing to its high production cost.
Therefore, efforts have been made to devise strategies for the effective and
inexpensive production of bacterial cellulose, these include testing various
waste materials such as fruit juices, industrial wastes, and food wastes (Islam
et al., 2017). The cost of the carbon source plays a key role in
bacterial cellulose production costs. Low-cost carbon sources such as glycerol
must be obtained as a byproduct of biodiesel production, and grape bagasse from
regional wine production for example. The volumetric productions (g/l) and the
yield (g/g) of bacterial cellulose in a culture medium by replacing glucose
with other carbon sources show that the production of this type of cellulose
with glucose has a cost of 0.22-0.24 U$D/kg, the cane molasses of 0.12 U$D/kg,
commercial glycerol 0.46-0.75 U$D/kg and glycerol from biodiesel 0.04-0.15
U$D/kg (Vázquez et al., 2013).
In Latin America with a volume produced of 6,152 thousand tons of
cellulose, there are profits of 1,486 million dollars. Between 2002 and early
2008, the price of pulp increased from $350 to $755 per ton, an increase of
116%. From the second semester of 2008, the price of cellulose started to fall,
reaching 475 dollars at the beginning of 2009, that is, a fall of 37% (Chapela,
2012).
CONCLUSIONS
Papermaking has
caused several negative impacts on the environment derived from deforestation,
such as habitat loss, biodiversity, soil erosion, and reduction of carbon
dioxide (CO2) fixation, thus suggesting the need to develop sustainable alternatives
to counteract these impacts, one of these alternatives is bacterial cellulose.
Gluconacetobacter
xylinus has great similarities with plant cellulose
(used for papermaking). It has been determined that bacterial cellulose is
structurally and chemically equal to plant cellulose, however, it has better
characteristics (high purity, high water absorption capacity and mechanical
resistance, and high degree of polymerization) which makes it a feasible
alternative for paper manufacturing, avoiding environmental damage caused by
the processes of paper production, mainly deforestation, debarking, screening,
and bleaching. Therefore, the knowledge of the growth conditions of G.
xylinus, its metabolism, formation, and excretion of bacterial cellulose,
and the structure of the synthesized cellulose, is decisive in knowing which
reactor to use to achieve maximum production of bacterial cellulose.
Despite its great
potential as an alternative to plant cellulose for papermaking, producing
bacterial cellulose is expensive; however, we must measure what conventional
paper manufacturing costs environmentally. Although the manufacture of paper
with plant cellulose is indeed more economical, this leads to severe impacts on
the environment. Therefore, it is considered necessary to continue making
efforts to design strategies for the economical production of bacterial
cellulose and to achieve sustainable paper manufacturing.
ACKNOWLEDGMENTS
The authors acknowledge support from
the project PROFAPI 2022_0001.
SOURCE OF FINANCING. No external financing.
Competing
interests. The authors have declared that no competing interests exist.
REFERENCES
Area, M. C. (2008). Panorama de la industria de celulosa y
papel en Iberoamérica 2008. Red Iberoamericana de Docencia e Investigación
en Celulosa y Papel-Riadicypp. Misiones, Argentina.
Aswini, K., Gopal, N. O. & Uthandi, S. (2020). Optimized
culture conditions for bacterial cellulose production by Acetobacter
senegalensis MA1. BMC Biotechnology, 20(1), 46. doi:10.1186/s12896-020-00639-6.
Baird, C. & Cain, M. (2018). Química ambiental. 2da ed. Reverté. https://books.google.com.mx/books?id=59zeDwAAQBAJ&printsec=frontcover&hl=en&source=gbs_ge_summary_r&cad=0#v=onepage&q&f=trueBajpai
Pratima. (2017).
Pulp and paper industry. Emerging Wastewater Treatment Technologies. TNQ Books
and Journals Elsevier. ISBN: 9780128110997.
Barrera-Martínez, C. L., Meléndez-Rentería, N. P., De León-Zapata,
M. A., Salinas-Jasso, T. A., Aguilar-González, C. N. & Laredo-Alcalá, E. I.
(2021). Polímeros de origen microbiano con aplicaciones agroindustriales.
International Journal of Research and Technological Innovation.
https://riiit.com.mx/apps/site/files_v2450/polmeros_uadec._3_riiit_div_ene-feb_2022.pdf.
Boeykens, S. (2006). Procesos para la producción de papel y
pulpa: de la naturaleza a la mesa. Encrucijadas, 38. Universidad de Buenos
Aires. Repository Institutional of the University of Buenos Aires. http://repositoriouba.sisbi.uba.ar/gsdl/collect/encruci/index/assoc/HWA_416.dir/416.PDF.
Carpintero, C. S. (2019). El agua en la industria de la celulosa y
el papel, bajo el signo de la eficiencia. Retema: Revista técnica de medio
ambiente, 32(215), 38-45.
Carreño Pineda, L. D., Caicedo Mesa, L. A. & Martínez Riascos
C.A. (2012). Técnicas de fermentación y aplicaciones de la celulosa bacteriana:
una revisión. Engineering and Science Journal, 8(16), 307-335. EAFIT
University.
Centro de Estudios para el Desarrollo Rural Sustentable y la
Soberanía Alimentaria (CEDRSSA) (2019). La actividad forestal en México,
estrategias y acciones contra la deforestación. Legislative Palace of San
Lázaro. México.
Chapela, G. (2012). Problemas y oportunidades en el mercado
para las empresas sociales forestales en México. Universidad Autónoma de
Chapingo.
Chávez-Pacheco, J. L., Yee, S. M., Zentella, M. C. & Marván,
E. E. (2004). Celulosa bacteriana en gluconacetobacter xylinum:
biosíntesis y aplicaciones. Tip Specialized Magazine in Chemical-Biological
Sciences, 7(1), 18-25. https://www.redalyc.org/articulo.oa?id=43270103
Coordinación General de Producción y Productividad de la Comisión
Nacional Forestal (CONAFORT) (2013). Bosques, cambio climático y REDD+ en
México. Guía básica. Área de Proyectos y Mercados Forestales de Carbono.
México. URL:
http://www.conafor.gob.mx:8080/documentos/docs/35/4034Gu%C3%ADa%20B%C3%A1sica%20de%20Bosques,%20Cambio%20Clim%C3%A1tico%20y%20REDD_%20.pdf
Costa, A. F., Almeida, F. C., Vinhas, G. M. & Sarubbo, L. A.
(2017). Production
of bacterial cellulose by Gluconacetobacter hansenii using corn steep liquor as
nutrient sources. Frontiers in microbiology, 8, 2027. doi: 10.3389/fmicb.2017.02027
Curilla Paucar, K. N. & Dias Huamani, D. F. (2020). Una revisión del uso de la celulosa
vegetal en los materiales de construcción: una perspectiva de sostenibilidad
ambiental en países desarrollados. (Bachelor’s Thesis). Universidad César Vallejo, Perú. https://hdl.handle.net/20.500.12692/60205
Domínguez Ríos, M. D. C., Hernández Contreras, R. G., & Medina
Hernández, R. M. (2017). Innovación Y Sustentabilidad de la Industria de Papel
En México (Innovation and Sustainability in the Mexico Paper Industry). Global Business Journal, 5(5), 87-97. https://ssrn.com/abstract=2916353
Francés Gómez, M. (2022). Evaluación de derivados de residuos agrícolas como materiales de
refuerzo en la producción de papel para embalaje a partir de fibras
secundarias. (Bachelor’s
Thesis Universidad Zaragoza). Zaragoza. https://zaguan.unizar.es/record/112295/files/TAZ-TFG-2022-184.pdf
Ferro, F., Ferró, P. & Ferró, A. (2019). Temporary distribution of acute
diarrheal diseases, its relationship with temperature and residual chlorine in
drinking water in the city of Puno, Peru. Rev. investig. Altoandin. 21(1).
scielo.org.pe/scielo.php?
Gorgieva, S. & Trček, J. (2019). Bacterial cellulose:
Production, modification and perspectives in biomedical applications. Nanomaterials, 9(10), 1352. doi:10.3390/nano9101352
Greenpeace (2004). El papel. Cómo reducir el consumo y
optimizar el uso y reciclaje de papel. http://archivo-es.greenpeace.org/espana/Global/espana/report/other/el-papel.pdf
Guinea Nava, M. (2016). Design of a pilot plant for the production
of Bacterial Cellulose. (Bachelor's thesis, Universitat Politècnica de
Catalunya). Available from http://hdl.handle.net/2117/98499
Gutiérrez Vélez, V.H. & Lopera Arango, G. J. (2001). Valoración
económica de la fijación de carbono en plantaciones tropicales de Pinus patula.
Medición y monitoreo de la captura de carbono en ecosistemas forestales. Valdivia,
Chile. Universidad Nacional de Colombia.
Haggith, M. & Martin, J. (2018). The state of the global paper industry. Environmental
Paper Network, 5.
environmentalpaper.org/wp-content/uploads/2018/04/StateOfTheGlobalPaperIndustry2018_FullReport-Final-1.pdf.
Herrera, C. & Amurrio, D. (2021). Cellulose acetate from
Viguiera tucumanensis. RevActaNova, 10 (1), 96-105. scielo.org.bo/scielo.php?pid=S1683-07892021000100006&script=sci_arttext
Islam, M. U., Ullah, M. W., Khan, S., Shah, N. & Park, J. K.
(2017). Strategies for cost-effective and enhanced production of bacterial
cellulose. International
Journal of Biological Macromolecules 102, 1166–1173. doi:10.1016/j.ijbiomac.2017.04.110
Jiménez
Pérez, J., Telles Antonio, R., Alanís Rodríguez, E., Yerena Yamallel, J. I.,
García García, D. A. & Gómez Cárdenas, M. (2020). Estimación del carbono
almacenado en una plantación de Tectona grandis L. f. mediante
ecuaciones alométricas. Revista Mexicana de Ciencias Forestales, 11(57),
32-56. doi:10.29298/rmcf.v11i57.550
Jónsson,
J. Ö. G., Davíðsdóttir, B. & Nikolaidis, N. P.
(2017). Valuation
of Soil Ecosystem Services. In S. Banwart & D. Sparks (Eds.), Advances
in Agronomy, Vol. 142, pp. 353–384. https://doi.org/10.1016/bs.agron.2016.10.011
Jónsson K., Kurzawa, M., Jędrzejczak-Krzepkowska, M., Ludwicka,
K., Krawczyk, M., Migdalski, A., Kacprzak, M. M., Loska, D., Krystynowicz, A.
& Bielecki, S. (2014). Complete genome sequence of Gluconacetobacter
xylinus E25 strain—Valuable and effective producer of bacterial nanocellulose. Journal
of Biotechnology, 176, 18-19. doi:10.1016/j.jbiotec.2014.02.006
Lahiri, D., Nag, M., Dutta, B., Dey, A., Sarkar, T., Pati, S.,
Edinur, H. A., Abdul Kari, Z., Mohd Noor, N. H. & Ray, R. R. (2021).
Bacterial Cellulose: Production, Characterization, and Application as
Antimicrobial Agent. International Journal of Molecular Sciences, 22(23),
12984. doi:10.3390/ijms222312984
Lera Santín, A. (2011). Aplicaciones enzimáticas en procesos de
conservación y restauración de obras de arte. Consolidación de celulosa.
Servicio Editorial de la Universidad del País Vasco/Euskal Herriko
Unibertsitatearen Argitalpen Zerbitzua. http://hdl.handle.net/10810/14292
Liu, C. L. C., Kuchma, O., & Krutovsky, K. V. (2018a).
Mixed-species versus monocultures in plantation forestry: Development,
benefits, ecosystem services and perspectives for the future. Global Ecology
and Conservation, 15, e00419. doi:10.1016/j.gecco.2018.e00419
Liu, M., Liu, L., Jia, S., Li, S., Zou, Y. & Zhong, C. (2018b).
Complete genome analysis of Gluconacetobacter xylinus CGMCC 2955 for
elucidating bacterial cellulose biosynthesis and metabolic regulation. Sci
Rep, 8 (1), 6266. doi:10.1038/s41598-018-24559-w
López, H. G., Vaides, E. E. & Alvarado, A. (2018). Evaluation
of carbon fixed in the aerial biomass of teak plantations in Chahal, Alta
Verapaz, Guatemala. Agronomía
Costarricense, 42(1),
137-153. doi:10.15517/rac.v42i1.32201
Merizalde, E., Montenegro, L., & Cabrera, M. (2019). Estudio
de un sistema de tratamiento de aguas residuales provenientes de una industria
de papel. Revista politécnica, 43(1), 7-14.
Mues, F., Rolón, J. N. & Rodriguez, M. E. (2018).
Determinación de Costos para la Producción de Celulosa mediante Método Kraft y
Mecánico. Revista Tecnología y Ciencia, (32), 307–318. https://rtyc.utn.edu.ar/index.php/rtyc/article/view/92
National Oceanic and Atmospheric Administration (NOAA). (2016). NOAA’s
annual greenhouse gas index. Earth System Research Laboratory Global Monitoring
Division, NOAA. https://gml.noaa.gov/aggi/
Pecoraro, É., Manzani, D., Messaddeq, Y. & Ribeiro, S. J. L.
(2007). Bacterial
Cellulose from Glucanacetobacter xylinus: Preparation, Properties and
Applications. In Monomers, Polymers and Composites from Renewable Resources. Elsevier, 369–383.. doi:10.1016/B978-0-08-045316-3.00017-X
Perna, O. (2013). Evaluación de la producción de celulosa por
Acetobacter xylinum ifo en presencia de melaza de caña bajo condiciones
estáticas y/o de flujo de aire intermitente. (Master's Thesis. Universidad
de la Sabana). Bogotá DC, Colombia.
Prada Ramírez, H.A. (2014). Papel del segundo mensajero c-di-GMP
en Pseudomonas syringae pv. tomato DC3000. Granada. Universidad de
Granada. URI http://hdl.handle.net/10481/34427.
Rojas Pérez, H. L., Díaz Vásquez, M. A., Muro Exebio, I. del R.
& Díaz Manchay, R. J. (2020). Sostenibilidad ambiental de la práctica
clínica, una nueva visión para enfermería. ACC CIETNA: Journal of the School of
Nursing, 7(1),
116-125. doi:10.35383/cietna.v7i1.353
Romero Conrado, A. R., Suárez Agudelo, E. A., Macías Jiménez, M.
A., Gómez Charris, Y. & Lozano Ayarza, L. P. (2017). Diseño experimental
para la obtención de compost apto para uso agrícola a partir de lodo papelero
Kraft. Spaces Journal. 38(28).
Santos de Dios, S. M. (2015). Aplicación de la celulosa
bacteriana a la restauración del patrimonio bibliográfico y documental en papel.
(Bachelor's tesis). Universidad Politécnica de Madrid. http://oa.upm.es/39035/1/07_2015_SARA_MARIA_SANTOS_DE_DIOS.pdf
Santos, S. M., Carbajo, J. M., Gómez, N.,
Quintana, E., Ladero, M., Sánchez, A., Chinga-Carrasco, G. & Villar, J. C.
(2016). Use of
bacterial cellulose in degraded paper restoration. Part II: application on real
samples. Journal of
Materials Science, 51(3),
1553–1561. doi:10.1007/s10853-015-9477-z
Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT).
(2016). Informe de la situación del medio ambiente en México; Compendio de
estadísticas ambientales, indicadores clave, de desempeño ambiental y de crecimiento
verde. Edición
2015.
Schmid, J., Sieber, V. & Rehm, B. (2015). Bacterial
exopolysaccharides: biosynthesis pathways and engineering strategies. Frontiers in Microbiology, 6,496. doi:10.3389/fmicb.2015.00496.
Teschke, K. & Demers, P. (2001). Industria del papel y de la
pasta de papel sectores basados en recursos biológicos. Enciclopedia de
salud y seguridad en el trabajo III, 72.1-71.21. https://www.virtualpro.co/biblioteca/industria-del-papel-y-de-la-pasta-del-papel
Tuya Salas, J., Gutiérrez Moreno, S. & Merino Rafael, F.
(2021). Ensayos preliminares para producción de celulosa por bacterias aisladas
de caña de azúcar. Revista de La Sociedad Química Del Perú, 87(2),
128–136. doi:10.37761/rsqp.v87i2.339
Valdovinos, N. & Franco, J. (2016). Occupational health in a
paper company in the State of Mexico. Revista Cubana de Salud y Trabajo, 17(2), 27-35.
Vázquez, A., Foresti, M. L., Cerrutti, P. & Galvagno, M.
(2013). Bacterial
Cellulose from Simple and Low Cost Production Media by Gluconacetobacter
xylinus. Journal of Polymers and the Environment, 21(2), 545–554. doi:10.1007/s10924-012-0541-3
Wang, J., Tavakoli, J. & Tang, Y. (2019). Bacterial cellulose
production, properties and applications with different culture methods – A
review. Carbohydrate Polymers, 219, 63–76. doi:10.1016/j.carbpol.2019.05.008
![]() , Ana Lucia Flores García1
, Ana Lucia Flores García1![]() , Magda Daniela Torres Portillo1
, Magda Daniela Torres Portillo1![]() , Maria Fernanda Ávila Mascareño1
, Maria Fernanda Ávila Mascareño1![]() , Karla Patricia Cota García1
, Karla Patricia Cota García1![]() , Andrea Denisse Martínez Vidales1
, Andrea Denisse Martínez Vidales1![]() , Fannie Isela Parra Cota2
, Fannie Isela Parra Cota2![]() , Sergio de los Santos Villalobos1*
, Sergio de los Santos Villalobos1*![]() 1Instituto Tecnológico de Sonora. Sonora, México. 2Campo
Experimental Norman E. Borlaug (CENEB). Instituto Nacional de Investigaciones
Forestales, Agrícolas y Pecuarias (INIFAP). Sonora, México *Autor de correspondencia:
sergio.delossantos@itson.edu.mx.com.
1Instituto Tecnológico de Sonora. Sonora, México. 2Campo
Experimental Norman E. Borlaug (CENEB). Instituto Nacional de Investigaciones
Forestales, Agrícolas y Pecuarias (INIFAP). Sonora, México *Autor de correspondencia:
sergio.delossantos@itson.edu.mx.com.